近日,权威医学期刊《新英格兰医学杂志》(NEJM)在线发表了一篇关于新型冠状病毒(2019-nCoV)的论文,向外界披露了美国首例确诊病例的整个诊疗过程和临床表现。
我们认为,这些信息对于帮助工作在一线的医护人员和普通大众都有着非常重要的意义。因此,甲骨易启动抗击疫情翻译专家小组,对论文的全文进行了精译,以便于为医务人员的临床诊疗工作提供借鉴,为人们的防疫自查提供参考。
该名患者在发病后的短短12天内被基本治愈:
前4天:2020年1月15日结束在武汉的探亲,返回美国便开始咳嗽,期间有两天的恶心呕吐史,但没有感到气短或胸痛。
第5天:入院第1天,接受了支持疗法,包括输注2升生理盐水和服用恩丹西酮缓解恶心。
第6-9天:住院的第2天至第5天,除了伴有心动过速的间歇性发热之外,患者的生命体征基本保持稳定。这段时间的治疗基本上采用支持性疗法。在症状处理方面,患者根据需要接受退热治疗,包括每隔4小时服用650毫克对乙酰氨基酚和每隔6小时服用600毫克布洛芬。
第10天:住院第6天,第四次胸片显示两肺存在基底条纹状浑浊,这一发现与非典型肺炎一致,听诊时发现两肺有罗音。临床医生根据“同情用药”原则,采用在研抗病毒疗法。
第11天:住院第7天,晚上停用万古霉素。
第12天:住院第8天,患者的临床病情有所改善。已停止输氧,当他呼吸环境空气时,血氧饱和度值提高到94%至96%。先前的双侧下叶罗音不再出现。患者的食欲有所改善,除了间歇性干咳和流鼻涕之外,没有其他症状。
SUMMARY
概述
An outbreak of novel coronavirus (2019-nCoV)that began in Wuhan, China, has spread rapidly, with cases now confirmed in multiple countries. We report the first case of 2019-nCoV infection confirmed in the United States and describe the identification, diagnosis, clinical course, and management of the case, including the patient’s initial mild symptoms at presentation with progression to pneumonia on day 9 of illness. This case highlights the importance of close coordination between clinician sand public health authorities at the local, state, and federal levels, as well as the need for rapid dissemination of clinical information related to the care of patients with this emerging infection.
一场始于中国武汉的新型冠状病毒(2019-nCoV)疫情已迅速蔓延,目前已在多个国家发现确诊病例。我们报道的是在美国确诊的首例2019-nCoV感染病例,并介绍该病例的鉴定、诊断、临床表现和治疗情况,包括患者最初出现的轻微症状并在发病第9天进展成肺炎的过程。本病例凸显出临床医生与地方、州和联邦各级公共卫生当局之间密切配合的重要性,以及快速宣传这种新发传染病相关临床护理信息的必要性。
ON DECEMBER 31, 2019, CHINA REPORTED A CLUSTEROF CASES OF pneumonia in people associated with the Huanan Seafood Whole sale Market in Wuhan, Hubei Province. On January 7, 2020, Chinese health authorities confirmed that this cluster was associated with a novel coronavirus, 2019-nCoV. Although cases were originally reported to be associated with exposure to the seafood market in Wuhan, current epidemiologic data indicate that person-to-person transmission of 2019-nCoV isoccurring.As of January 30, 2020, a total of 9976 cases had been reported in at least 21 countries,including the first confirmed case of 2019-nCoV infection in the United States, reported on January 20, 2020.Investigations are under way worldwide to better understand transmission dynamics and the spectrum of clinical illness. This report describes the epidemiologic and clinical features of the first case of 2019-nCoV infection confirmed in the United States.
2019年12月31日,中国报告了在湖北省武汉市华南海鲜批发市场相关人群中发现的一组肺炎病例。2020年1月7日,中国卫生部门证实该组群与2019-nCoV有关。虽然最初报道的病例与武汉海鲜市场接触有关系,但最新流行病学数据表明该2019-nCoV正在发生人际传播。截至2020年1月30日,已有至少21个国家报告了共计9,976例病例,其中包括2020年1月20日报告的美国首例2019-nCoV感染确诊病例。研究正在全球范围内展开,以便更好地了解该病毒的传播动力学和临床表现。本报告介绍了美国确诊的首例2019-nCoV感染的流行病学特征和临床特征。

CASE REPORT
病例报告
On January 19, 2020, a 35-year-old man presented to an urgent care clinic in Snohomish County, Washington, with a 4-day history of cough and subjective fever. On checking into the clinic, the patient put on a mask in the waiting room. After waiting approximately 20 minutes, he was taken into an examination room and underwent evaluation by a provider. He disclosed that he had returned to Washington State on January 15 after traveling to visit family in Wuhan, China. The patient stated that he had seen a health alert from the U.S. Centers for Disease Control and Prevention (CDC) about the novel coronavirus outbreak in China and, because of his symptoms and recent travel, decided to see a health care provider.
2020年1月19日,一名35岁男子前往华盛顿州斯诺霍米什县的一家急诊诊所就医,他已经咳嗽了4天,并患有主观性发热。该患者在诊所挂号和候诊时就已戴好口罩。等待约20分钟后,他进入检查室并接受医生的检查。他透露自己前往中国武汉探亲后于1月15日回到华盛顿州。该患者表示,他已经看到美国疾病控制与预防中心(CDC)发出的关于中国爆发2019-nCoV的健康警报,考虑到自身出现的症状和近期行程,他决定就医。
Apart from a history of hypertriglyceridemia, the patient was an otherwise healthy nonsmoker. The physical examination revealed a body temperature of 37.2°C, blood pressure of 134/87 mm Hg, pulse of 110 beats per minute, respiratory rate of 16 breaths per minute, and oxygen saturation of 96% while the patient was breathing ambient air. Lung auscultation revealed rhonchi, and chest radiography was performed, which was reported as showing no abnormalities (Figure 1). A rapid nucleic acid amplification test (NAAT) for influenza A and B was negative. A nasopharyngeal swab specimen was obtained and sent for detection of viral respiratory pathogens by NAAT; this was reported back within 48 hours as negative for all pathogens tested, including influenza A and B, parainfluenza, respiratory syncytial virus, rhinovirus, adenovirus, and four common coronavirus strains known to cause illness in humans (HKU1, NL63, 229E, and OC43).
除了患有高甘油三酯血症病史之外,该患者是一名注重健康的不吸烟者。体检结果显示患者呼吸环境空气时的体温为37.2摄氏度,血压134/87毫米汞柱,心率每分钟110跳,呼吸频率每分钟16次,氧饱和度96%。患者在接受肺部听诊时发现有罗音,并接受了胸片检查,但报告显示没有异常(图1)。甲型和乙型流感的快速核酸扩增检测(NAAT)结果均为阴性。医务人员收集了患者的鼻咽拭子样本,并将其送去进行病毒性呼吸道病原体的检测(通过核酸扩增检测);所有病原体的检测报告在48小时内送回,结果均为阴性,包括甲型和乙型流感、副流感、呼吸道合胞病毒、鼻病毒、腺病毒和四种已知会导致人类疾病的常见冠状病毒株(HKU1、NL63、229E和OC43)。
Given the patient’s travel history, the local and state health departments were immediately notified. Together with the urgent care clinician, the Washington Department of Health notified the CDC Emergency Operations Center. Although the patient reported that he had not spent time at the Huanan seafood market and reported no known contact with ill persons during his travel to China, CDC staff concurred with the need to test the patient for 2019-nCoV on the basis of current CDC “persons under investigation” case definitions.8 Specimens were collected in accordance with CDC guidance and included serum and nasopharyngeal and oropharyngeal swab specimens. After specimen collection, the patient was discharged to home isolation with active monitoring by the local health department.
考虑到患者的旅行史,该诊所立即通知了当地及州卫生部门。华盛顿州卫生署与急诊临床医师一起通知了疾病预防控制中心(CDC)的应急行动中心。虽然患者称并未到过华南海鲜市场,在中国旅行期间也未曾与已知患病者有过接触,但疾病预防控制中心的工作人员一致认为需要按照目前疾病预防控制中心对“受调查人员”病例定义对患者进行2019-nCoV检测。8检测样本按照疾病预防控制中心的指导收集,包括血清、鼻咽和口咽拭子样本。样本收集好后,患者出院回家,并在当地卫生部门的严密监控下在家中进行自我隔离。
On January 20, 2020, the CDC confirmed that the patient’s nasopharyngeal and oropharyngeal swabs tested positive for 2019-nCoV by real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) assay. In coordination with CDC subject-matter experts, state and local health officials, emergency medical services, and hospital leadership and staff, the patient was admitted to an airborne-isolation unit at Providence Regional Medical Center for clinical observation, with health care workers following CDC recommendations for contact, droplet, and airborne precautions with eye protection.
2020年1月20日,疾病预防控制中心通过实时逆转录-聚合酶链反应(rRT-PCR)检测,证实患者的鼻咽和口咽拭子检测结果均呈现2019-nCoV阳性。在与疾病预防控制中心主题专家、州及地方卫生官员、紧急医学治疗服务部门以及医院领导和工作人员的配合下,患者被送入普罗维登斯地区医学治疗中心的空气隔离病房进行临床观察,所有医护人员遵循疾病预防控制中心提出的关于接触、飞沫、空气传播预防措施的建议,并佩戴眼部防护用品。
On admission, the patient reported persistent dry cough and a 2-day history of nausea and vomiting; he reported that he had no shortness of breath or chest pain. Vital signs were within normal ranges. On physical examination, the patient was found to have dry mucous membranes. The remainder of the examination was generally unremarkable. After admission, the patient received supportive care, including 2 liters of normal saline and ondansetron for nausea.
入院时,患者报称持续干咳,且有两天的恶心呕吐史,但没有感到气短或胸痛。生命体征也在正常范围之内。经体检,发现患者粘膜干燥。其余的检查结果都很平常。入院后,患者接受了支持疗法,包括输注2升生理盐水和服用恩丹西酮缓解恶心。
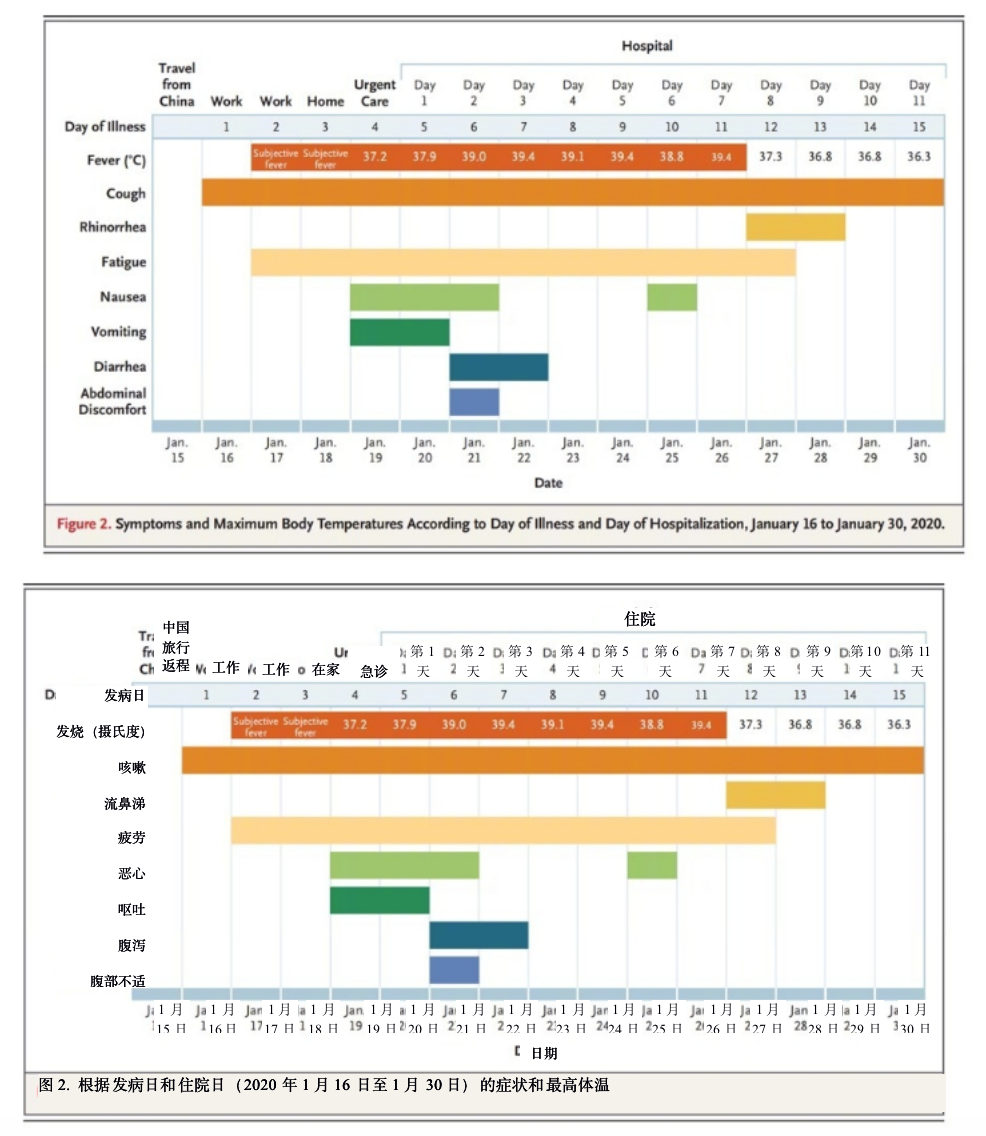
On days 2 through 5 of hospitalization (days 6 through 9 of illness), the patient’s vital signs remained largely stable, apart from the development of intermittent fevers accompanied by periods of tachycardia (Figure 2). The patient continued to report a nonproductive cough and appeared fatigued. On the afternoon of hospital day 2, the patient passed a loose bowel movement and reported abdominal discomfort. A second episode of loose stool was reported overnight; a sample of this stool was collected for rRT-PCR testing, along with additional respiratory specimens (nasopharyngeal and oropharyngeal) and serum. The stool and both respiratory specimens later tested positive by rRT-PCR for 2019-nCoV, whereas the serum remained negative.
住院的第2天至第5天(发病第6天至第9天),除了伴有心动过速的间歇性发热之外,患者的生命体征基本保持稳定(图2)。患者继续报称干咳,且感觉很疲劳。住院第2天的下午,患者出现腹泻现象,并感到腹部不适。患者在夜间发生第二次腹泻;此次腹泻的粪便样本被收集,同时再次收集呼吸道样本(鼻咽和口咽)以及血清,用于rRT-PCR检测。粪便和两个呼吸道样本的rRT-PCR检测结果均呈现2019-nCoV阳性,但血清检测结果仍然呈阴性。
Treatment during this time was largely supportive. For symptom management, the patient received, as needed, antipyretic therapy consisting of 650 mg of acetaminophen every 4 hours and 600 mg of ibuprofen every 6 hours. He also received 600 mg of guaifenesin for his continued cough and approximately 6 liters of normal saline over the first 6 days of hospitalization.
这段时间的治疗基本上采用支持性疗法。在症状处理方面,患者根据需要接受退热治疗,包括每隔4小时服用650毫克对乙酰氨基酚和每隔6小时服用600毫克布洛芬。在住院的前6天,他还因持续咳嗽服用了600毫克愈创甘油醚并输注了约6升生理盐水。
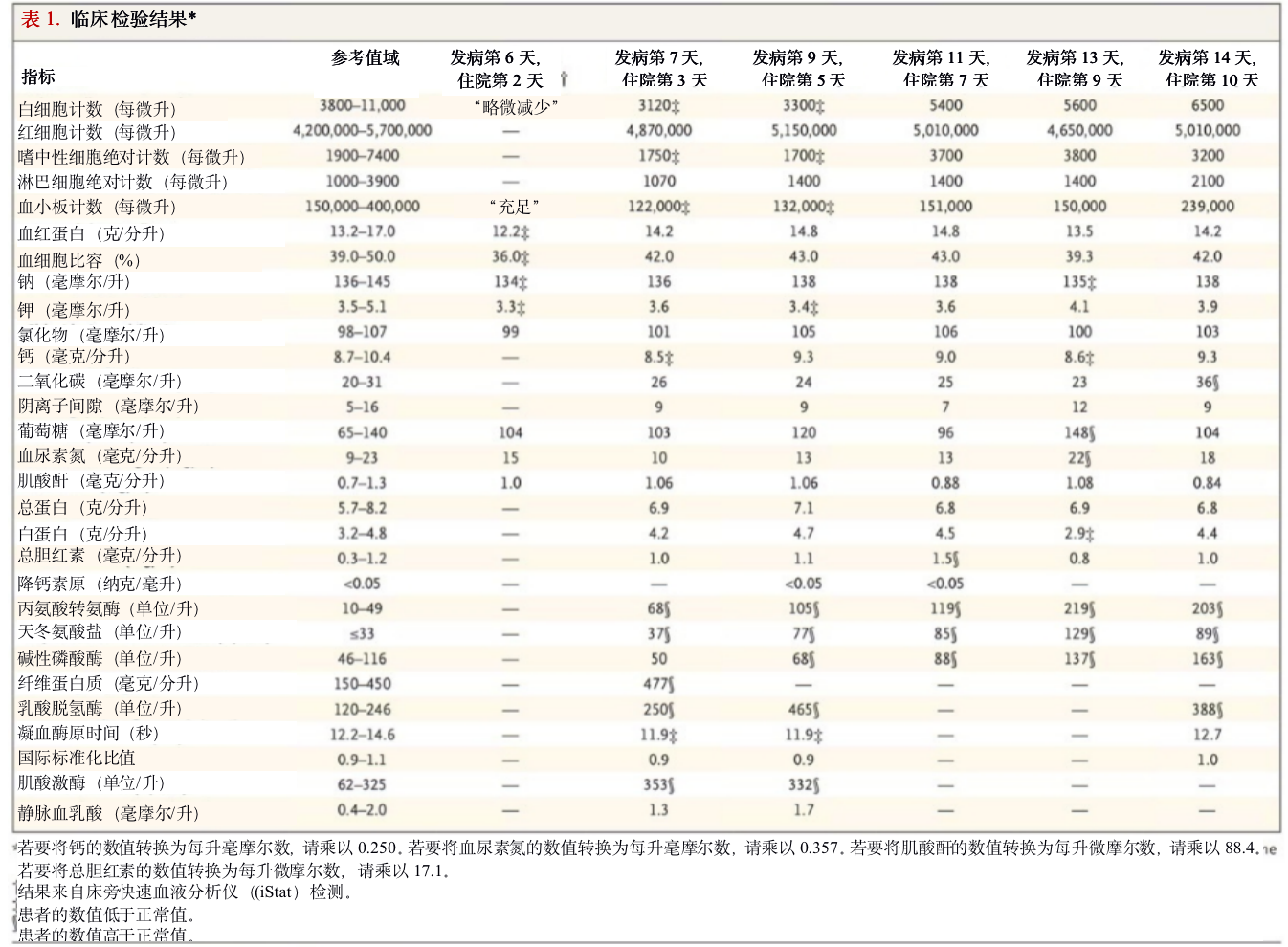
The nature of the patient isolation unit permitted only point-of-care laboratory testing initially; complete blood counts and serum chemical studies were available starting on hospital day 3. Laboratory results on hospital days 3 and 5 (illness days 7 and 9) reflected leukopenia, mild thrombocytopenia, and elevated levels of creatine kinase (Table 1). In addition, there were alterations in hepatic function measures: levels of alkaline phosphatase (68 U per liter), alanine aminotransferase (105 U per liter), aspartate aminotransferase (77 U per liter), and lactate dehydrogenase (465 U per liter) were all elevated on day 5 of hospitalization. Given the patient’s recurrent fevers, blood cultures were obtained on day 4; these have shown no growth to date.
患者隔离单元的性质最初只允许进行即时实验室测试;从住院第3天开始,可以进行完整的血液计数和血清化学研究。住院第3天和第5天(发病第7天和第9天)的实验室结果反映出白细胞减少、轻度血小板减少和肌酸激酶水平升高(表1)。此外,肝功能指标也有变化:住院第5天的碱性磷酸酶(68单位/升)、丙氨酸转氨酶(105单位/升)、天冬氨酸转氨酶(77单位/升)和乳酸脱氢酶(465单位/升)水平均有所升高。考虑到患者反复发烧,在第4天进行了血培养;到目前为止,相关指标没有任何增长。
A chest radiograph taken on hospital day 3 (illness day 7) was reported as showing no evidence of infiltrates or abnormalities (Figure 3). However, a second chest radiograph from the night of hospital day 5 (illness day 9) showed evidence of pneumonia in the lower lobe of the left lung (Figure 4). These radiographic findings coincided with a change in respiratory status starting on the evening of hospital day 5, when the patient’s oxygen saturation values as measured by pulse oximetry dropped to as low as 90% while he was breathing ambient air. On day 6, the patient was started on supplemental oxygen, delivered by nasal cannula at 2 liters per minute. Given the changing clinical presentation and concern about hospital-acquired pneumonia, treatment with vancomycin (a 1750-mg loading dose followed by 1 g administered intravenously every 8 hours) and cefepime (administered intravenously every 8 hours) was initiated.
住院第3天(发病第7天)拍摄的胸片显示没有浸润或异常迹象(图3)。然而,住院第5天(发病第9天)晚上的第二次胸片显示左肺下叶有肺炎迹象(图4)。这些X光检查结果与住院第5天晚上开始的呼吸系统状况变化相吻合,即当患者呼吸环境空气时,通过脉搏血氧仪测得的氧饱和度值跌至90%。第6天,患者开始接受补充氧气,以每分钟2升的速度通过鼻导管进行输氧。考虑到临床表现的变化和对医院获得性肺炎的担忧,开始使用万古霉素(1750毫克负荷剂量,随后每隔8小时静脉给药1克)和头孢吡肟(每隔8小时静脉给药一次)进行治疗。


On hospital day 6 (illness day 10), a fourth chest radiograph showed basilar streaky opacities in both lungs, a finding consistent with atypical pneumonia (Figure 5), and rales were noted in both lungs on auscultation. Given the radiographic findings, the decision to administer oxygen supplementation, the patient’s ongoing fevers, the persistent positive 2019-nCoV RNA at multiple sites, and published reports of the development of severe pneumonia3,4 at a period consistent with the development of radiographic pneumonia in this patient, clinicians pursued compassionate use of an investigational antiviral therapy. Treatment with intravenous remdesivir (a novel nucleotide analogue prodrug in development10,11) was initiated on the evening of day 7, and no adverse events were observed in association with the infusion. Vancomycin was discontinued on the evening of day 7, and cefepime was discontinued on the following day, after serial negative procalcitonin levels and negative nasal PCR testing for methicillin-resistant Staphylococcus aureus.
住院第6天(发病第10天),第四次胸片显示两肺存在基底条纹状浑浊,这一发现与非典型肺炎一致(图5),听诊时发现两肺有罗音。根据X光检查结果,医护人员决定给患者输氧,患者持续高烧,且多个部位持续呈阳性的2019-nCoV检测结果;再者,同时期已发表的重症肺炎3,4发展报告与该患者胸片中显示的肺炎发展情况相一致,因此,临床医生根据“同情用药”原则,采用在研抗病毒疗法。第7天晚上开始静脉输注remdesivir(一种正在研发中的新型核苷酸类似物前药10,11),未观察到与输注相关的不良事件。在第7天晚上停用万古霉素,次日,在对耐甲氧西林金黄色葡萄球菌进行连续的降钙素原阴性水平和鼻腔阴性PCR测试后,停用头孢吡肟。
On hospital day 8 (illness day 12), the patient’s clinical condition improved. Supplemental oxygen was discontinued, and his oxygen saturation values improved to 94 to 96% while he was breathing ambient air. The previous bilateral lower-lobe rales were no longer present. His appetite improved, and he was asymptomatic aside from intermittent dry cough and rhinorrhea. As of January 30, 2020, the patient remains hospitalized. He is afebrile, and all symptoms have resolved with the exception of his cough, which is decreasing in severity.
住院第8天(发病第12天),患者的临床病情有所改善。已停止输氧,当他呼吸环境空气时,血氧饱和度值提高到94%至96%。先前的双侧下叶罗音不再出现。患者的食欲有所改善,除了间歇性干咳和流鼻涕之外,没有其他症状。截至2020年1月30日,患者仍在住院治疗,但已经退烧,除咳嗽外,其他症状均已消退,咳嗽的严重程度也在减轻。


METHODS
SPECIMEN COLLECTION
Clinical specimens for 2019-nCoV diagnostic testing were obtained in accordance with CDC guidelines.12 Nasopharyngeal and oropharyngeal swab specimens were collected with synthetic fiber swabs; each swab was inserted into a separate sterile tube containing 2 to 3 ml of viral transport medium. Serum was collected in a serum separator tube and then centrifuged in accordance with CDC guidelines. The urine and stool specimens were each collected in sterile specimen containers. Specimens were stored between 2°C and 8°C until ready for shipment to the CDC. Specimens for repeat 2019-nCoV testing were collected on illness days 7, 11, and 12 and included nasopharyngeal and oropharyngeal swabs, serum, and urine and stool samples.
方法
样本采集
新型冠状病毒诊断检测的临床样本采集遵循CDC指南12的要求。使用合成纤维拭子采集了鼻咽拭子和口咽拭子样本,将各个拭子分别放入含有2至3毫升病毒运送培养液的无菌试管。根据CDC指南的要求用血清分离管采集血清,然后进行离心。用无菌样本容器分别采集尿液样本和粪便样本。在运往CDC前,样本的储存温度为20C至80C。在患病第7、11及12天进行了2019-nCoV复测样本的采集,包括鼻咽拭子、口咽拭子、血清、尿液和大便样本。
DIAGNOSTIC TESTING FOR 2019-NCOV
Clinical specimens were tested with an rRT-PCR assay that was developed from the publicly released virus sequence. Similar to previous diagnostic assays for severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), it has three nucleocapsid gene targets and a positive control target. A description of this assay13 and sequence information for the rRT-PCR panel primers and probes14 are available on the CDC Laboratory Information website for 2019-nCoV.
2019-nCoV的诊断检测
根据公开发布的病毒序列开发出了rRT-PCR测定法,用该测定法检测了临床样本。与以往针对急性重症呼吸综合征冠状病毒(SARS-CoV)和中东呼吸综合征冠状病毒(MERS-CoV)的诊断检测相似,2019-nCoV的诊断检测有三个核衣壳基因靶点及一个阳性对照靶点。该测定法13的说明及rRT-PCR引物和探针14的序列信息,请参见美国CDC实验室信息网关于2019-nCoV的内容。
GENETIC SEQUENCING
On January 7, 2020, Chinese researchers shared the full genetic sequence of 2019-nCoV through the National Institutes of Health GenBank database and the Global Initiative on Sharing All Influenza Data (GISAID) database; a report about the isolation of 2019-nCoV was later published. Nucleic acid was extracted from rRT-PCR–positive specimens (oropharyngeal and nasopharyngeal) and used for whole-genome sequencing on both Sanger and next-generation sequencing platforms (Illumina and MinIon). Sequence assembly was completed with the use of Sequencher software, version 5.4.6 (Sanger); minimap software, version 2.17 (MinIon); and freebayes software, version 1.3.1 (MiSeq). Complete genomes were compared with the available 2019-nCoV reference sequence (GenBank accession number NC_045512.2).
基因测序
2020年1月7日,中国研究人员在美国卫生研究院基因库数据库和全球流感数据共享计划(GISAID)数据库共享了2019-nCoV的完整基因序列;之后又发表了分离2019-nCoV的报告。从rRT-PCR阳性样本(口咽部和鼻咽部)提取出核酸,并用桑格测序法和新一代测序平台(Illumina和MinIon)进行了全基因组测序。序列组装采用Sequencer软件5.4.6版(桑格);minimap软件2.17版(MinIon)和freebayes软件1.3.1版(MiSeq)。将完整基因组与已发布的2019-nCoV参考序列(GenBank登录号NC_045512.2)进行了比较。
RESULTS
SPECIMEN TESTING FOR 2019-NCOV
The initial respiratory specimens (nasopharyngeal and oropharyngeal swabs) obtained from this patient on day 4 of his illness were positive for 2019-nCoV (Table 2). The low cycle threshold (Ct) values (18 to 20 in nasopharyngeal specimens and 21 to 22 in oropharyngeal specimens) on illness day 4 suggest high levels of virus in these specimens, despite the patient’s initial mild symptom presentation. Both upper respiratory specimens obtained on illness day 7 remained positive for 2019-nCoV, including persistent high levels in a nasopharyngeal swab specimen (Ct values, 23 to 24). Stool obtained on illness day 7 was also positive for 2019-nCoV (Ct values, 36 to 38). Serum specimens for both collection dates were negative for 2019-nCoV. Nasopharyngeal and oropharyngeal specimens obtained on illness days 11 and 12 showed a trend toward decreasing levels of virus. The oropharyngeal specimen tested negative for 2019-nCoV on illness day 12. The rRT-PCR results for serum obtained on these dates are still pending.
结果
2019-NCOV的样本检测
在患者发病第4天采集的初始呼吸道样本(鼻咽拭子和口咽拭子)呈2019-nCoV 阳性(表2)。虽然患者的初期症状较轻,发病第4天的低循环阈值(Ct)(鼻咽样本为18-20,口咽样本为21-22)表明,这些样本中的病毒载量较高。发病第7天采集的两份上呼吸道样本仍呈2019-nCoV阳性,鼻咽拭子样本的病毒载量仍然高(循环阈值为23-24)。发病第7天采集的大便样本也呈2019-nCoV阳性(循环阈值为36-38)。在两个采样日采集的血清样本均呈2019-nCoV阴性。发病第11天和第12天采集的鼻咽样本和口咽样本显示病毒载量呈下降趋势。患病第12天采集的口咽样本呈2019-nCoV阴性。在上述日期采集的血清样本的rRT-PCR检测尚未出结果。

GENETIC SEQUENCING
The full genome sequences from oropharyngeal and nasopharyngeal specimens were identical to one another and were nearly identical to other available 2019-nCoV sequences. There were only 3 nucleotides and 1 amino acid that differed at open reading frame 8 between this patient’s virus and the 2019-nCoV reference sequence (NC_045512.2). The sequence is available through GenBank (accession number MN985325).
基因测序
口咽样本和鼻咽样本的全基因组序列完全相同,与已发布的其他2019-nCoV序列几乎相同。该患者的病毒和2019-nCoV参考序列(NC_045512.2)之间,只有开放阅读框8处的3个核苷酸和1个氨基酸存在差异。该参考序列来自GenBank(登录号MN985325。
DISCUSSION
Our report of the first confirmed case of 2019-nCoV in the United States illustrates several aspects of this emerging outbreak that are not yet fully understood, including transmission dynamics and the full spectrum of clinical illness. Our case patient had traveled to Wuhan, China, but reported that he had not visited the wholesale seafood market or health care facilities or had any sick contacts during his stay in Wuhan. Although the source of his 2019-nCoV infection is unknown, evidence of person-to-person transmission has been published. Through January 30, 2020, no secondary cases of 2019-nCoV related to this case have been identified, but monitoring of close contacts continues.
讨论
我们报道的美国首例2019-nCoV 确诊病例说明此新暴发疫情尚有几方面未完全清楚,包括传播动力学和临床疾病的发展全貌。此病例患者曾去过中国武汉,但在武汉期间未去过海鲜批发市场和医学治疗机构,也未接触过任何病患。尽管该病患的2019-nCoV感染来源不明,但已公开了人传人的证据。截至2020年1月30日,尚未发现与该病例相关的2019-nCoV继发病例,但仍在继续监控其密切接触者。
Detection of 2019-nCoV RNA in specimens from the upper respiratory tract with low Ct values on day 4 and day 7 of illness is suggestive of high viral loads and potential for transmissibility. It is notable that we also detected 2019-nCoV RNA in a stool specimen collected on day 7 of the patient’s illness. Although serum specimens from our case patient were repeatedly negative for 2019-nCoV, viral RNA has been detected in blood in severely ill patients in China.4 However, extrapulmonary detection of viral RNA does not necessarily mean that infectious virus is present, and the clinical significance of the detection of viral RNA outside the respiratory tract is unknown at this time.
发病第4天和第7天上呼吸道样本中检出2019-nCoV核糖核酸,且循环阈值较低,提示病毒载量高,有潜在的传播性。值得注意的是,我们还在发病第7天采集的大便样本中也检测到了2019-nCoV核糖核酸。尽管该病患血清样本屡次呈2019-nCoV阴性,但在中国,重症患者血液内已检测到病毒核糖核酸4。不过,肺外检测到病毒核糖核酸不一定意味着存在传染性病毒,目前还不清楚呼吸道外检测到病毒核糖核酸的临床意义。
Currently, our understanding of the clinical spectrum of 2019-nCoV infection is very limited. Complications such as severe pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS), and cardiac injury, including fatal outcomes, have been reported in China.4,18,20 However, it is important to note that these cases were identified on the basis of their pneumonia diagnosis and thus may bias reporting toward more severe outcomes.
目前,我们对2019新型冠状病毒感染的临床情况了解非常有限。中国已报告了并发症,如重度肺炎、呼吸衰竭、急性呼吸窘迫综合征(ARDS)和心脏损伤,包括死亡4,18,20。不过,值得注意的是,这些病例是在肺炎诊断的基础上确定的,因此可能偏向报告更严重的结果。
Our case patient initially presented with mild cough and low-grade intermittent fevers, without evidence of pneumonia on chest radiography on day 4 of his illness, before having progression to pneumonia by illness day 9. These nonspecific signs and symptoms of mild illness early in the clinical course of 2019-nCoV infection may be indistinguishable clinically from many other common infectious diseases, particularly during the winter respiratory virus season. In addition, the timing of our case patient’s progression to pneumonia on day 9 of illness is consistent with later onset of dyspnea (at a median of 8 days from onset) reported in a recent publication.4 Although a decision to administer remdesivir for compassionate use was based on the case patient’s worsening clinical status, randomized controlled trials are needed to determine the safety and efficacy of remdesivir and any other investigational agents for treatment of patients with 2019-nCoV infection.
我们的病患最初表现为轻微咳嗽和间断性低热,发病第4天胸片无肺炎表现,在发病第9天时发展为肺炎。2019-nCoV感染病程早期,轻症患者的这些非特异性症状和体征在临床上可能与许多其他常见传染病无法区分,尤其是在呼吸道病毒感染高发的冬季。此外,该病患在患病第9天发展为肺炎,这一时间与最近发表的文章4中报道的迟发性呼吸困难(中位时间为发病第8天)相符。尽管因患者临床状况恶化而决定使用瑞德西韦进行同情用药,但需要进行随机对照试验以确定瑞德西韦及治疗2019-nCoV感染患者的其它试验药的安全性和疗效。
We report the clinical features of the first reported patient with 2019-nCoV infection in the United States. Key aspects of this case included the decision made by the patient to seek medical attention after reading public health warnings about the outbreak; recognition of the patient’s recent travel history to Wuhan by local providers, with subsequent coordination among local, state, and federal public health officials; and identification of possible 2019-nCoV infection, which allowed for prompt isolation of the patient and subsequent laboratory confirmation of 2019-nCoV, as well as for admission of the patient for further evaluation and management. This case report highlights the importance of clinicians eliciting a recent history of travel or exposure to sick contacts in any patient presenting for medical care with acute illness symptoms, in order to ensure appropriate identification and prompt isolation of patients who may be at risk for 2019-nCoV infection and to help reduce further transmission. Finally, this report highlights the need to determine the full spectrum and natural history of clinical disease, pathogenesis, and duration of viral shedding associated with 2019-nCoV infection to inform clinical management and public health decision making.
本文报道了美国首例报告的2019-nCoV感染患者的临床特征。该病例要点包括患者看到关于疫情的公共卫生警告后决定就医;当地医务人员了解到患者近期曾去过武汉,随后当地、州和联邦公共卫生官员彼此协调;识别出可能感染2019-nCoV,从而能够迅速隔离患者,随后实验室确认2019-nCoV,以及将患者收入院进一步检查和治疗。此病例报告凸显出临床医师询问因急性病症而就诊的患者的近期旅行史或与其他患者接触史的重要性,从而确保正确识别和迅速隔离可能感染2019-nCoV的患者,降低进一步传播风险。最后,此报告还强调,我们需要确定与2019-nCoV感染相关临床疾病的全部表现和自然病程、发病机制以及病毒释出体外的病毒散发期,从而指导临床治疗和公共卫生决策。
本文原文来源于NEJM.org,版权归原作者所有,译文由甲骨易提供。